Saturday, October 27, 2007
pill in the pocket AF
"Pill in the Pocket" for Selected Patients with Atrial Fibrillation
A trial of self-treatment with antiarrhythmic drugs in highly selected outpatients shows promise for this strategy.
Patients with infrequent, relatively well-tolerated atrial fibrillation (AF) might not warrant prophylactic antiarrhythmic drug therapy or catheter ablation, but they often require emergency attention for symptomatic episodes. To test outpatient single-dose antiarrhythmic drug use for terminating acute episodes, researchers in Italy studied patients who presented to the emergency department with hemodynamically well-tolerated recent-onset AF. Patients with structural heart disease, conduction-system disease, or major noncardiac comorbidities were excluded, leaving 268 patients who were given single oral doses of flecainide or propafenone and were monitored for at least 8 hours. Fifty-eight patients then were excluded for side effects or lack of efficacy, and the remaining 210 were instructed on outpatient use of the antiarrhythmic agent.
During a mean follow-up of 15 months, 165 patients experienced 618 arrhythmic episodes; during 92% of these, patients treated themselves with single doses of the antiarrhythmic drug at an average of 36 minutes after symptom onset. Palpitations stopped in 94% of episodes after an average of 113 minutes. The drug was effective in terminating all arrhythmic episodes in 84% of patients, and monthly ED visits and hospitalizations were significantly less common after enrollment than during the previous year. Twelve patients had adverse effects, including 1 episode of rapid atrial flutter.
Comment: Electrophysiologists have long been aware that flecainide and propafenone can convert acute episodes of atrial fibrillation, but this is one of the first large evaluations in outpatients. Nevertheless, only practitioners who are thoroughly familiar with the use of these agents should prescribe them, and careful screening for underlying heart disease is imperative.
— Kirsten E. Fleischmann, MD, MPH
Published in Journal Watch General Medicine January 11, 2005
NEJM vol351 Paolo Alboni et al.
ABSTRACT Background In-hospital administration of flecainide and propafenone in a single oral loading dose has been shown to be effective and superior to placebo in terminating atrial fibrillation. We evaluated the feasibility and the safety of self-administered oral loading of flecainide and propafenone in terminating atrial fibrillation of recent onset outside the hospital.
Methods We administered either flecainide or propafenone orally to restore sinus rhythm in 268 patients with mild heart disease or none who came to the emergency room with atrial fibrillation of recent onset that was hemodynamically well tolerated. Of these patients, 58 (22 percent) were excluded from the study because of treatment failure or side effects. Out-of-hospital self-administration of flecainide or propafenone — the "pill-in-the-pocket" approach — after the onset of heart palpitations was evaluated in the remaining 210 patients (mean age [±SD], 59±11 years).
Results During a mean follow-up of 15±5 months, 165 patients (79 percent) had a total of 618 episodes of arrhythmia; of those episodes, 569 (92 percent) were treated 36±93 minutes after the onset of symptoms. Treatment was successful in 534 episodes (94 percent); the time to resolution of symptoms was 113±84 minutes. Among the 165 patients with recurrences, the drug was effective during all the arrhythmic episodes in 139 patients (84 percent). Adverse effects were reported during one or more arrhythmic episodes by 12 patients (7 percent), including atrial flutter at a rapid ventricular rate in 1 patient and noncardiac side effects in 11 patients. The numbers of monthly visits to the emergency room and hospitalizations were significantly lower during follow-up than during the year before the target episode (P<0.001>
Conclusions In a selected, risk-stratified population of patients with recurrent atrial fibrillation, pill-in-the-pocket treatment is feasible and safe, with a high rate of compliance by patients, a low rate of adverse events, and a marked reduction in emergency room visits and hospital admissions.
Thursday, October 25, 2007
statins and AF and CRP
Statins and Atrial Fibrillation
Statins -- in addition to their lipid lowering effects -- may reduce inflammation and oxidative stress. Inflammation (documented by higher levels of C-reactive protein) appears to be involved in the early phase of electrical remodeling (even within 24 hours after AF initiation) and promotes persistence of AF. While inflammation may be a pathogenetic component of AF, the clinical role for antiinflammatory drugs still remains unclear.[4,5]
In clinical studies, new-onset AF was reduced by statin medication in patients with coronary heart disease or reduced left ventricular function. Sparse data, though, exist on secondary prevention of AF by statin medication. A single study documented lower AF recurrences under statins after cardioversion of lone AF patients,[8] but no study on secondary prevention in patients with structural heart disease is available. The study by Al Chekakie et al.[7] and another study by Richter et al.[9] failed to show any effect of statins in the recurrence rates after catheter ablation of mostly long-lasting AF. This "negative" result does not rule out that statins might prevent AF in a very early stage of the disease where electrical remodeling can be manipulated by inhibiting inflammation and oxidative stress.
C-reactive protein (CRP) has been found to be elevated early after initiation of AF and chronic CRP levels have been indicated to be higher in patients with AF and decrease after conversion to sinus rhythm.[5,6] CRP was not evaluated in the study by Al Chekakie et al.,[7] but in the study by Richter et al.[9] no difference in preablation CRP was found in patients already under statin therapy. In both studies, CRP was not used as prospective selection criterion for postinterventional statin medication. Experimental studies, however, support the hypothesis that CRP levels might identify a subgroup of patients who would benefit from adding antioxidative medication after ablation of AF.
AF
American heart Journal
posted 09-06.2007
Medscape gastroenterology on line
cardiology 25 oct 07
Abstract
Objectives: We evaluated the effect of angiotensin-converting enzyme (ACE) inhibitor ramipril on the incidence of atrial fibrillation (AF) in patients enrolled in the Heart Outcomes Prevention Evaluation trial.
Background: Atrial fibrillation is the most common arrhythmia affecting the general population and is associated with increased morbidity and mortality. Retrospective secondary analyses of some of the large trials of ACE inhibitors have suggested that ACE inhibitors may prevent AF.
Methods: We evaluated the occurrence of AF by reviewing the electrocardiogram tracings at entry, at 2 years, and at the end of the study, as well as hospitalizations among 8335 high-risk participants from the Heart Outcomes Prevention Evaluation study, ≥55 years, without known heart failure or left ventricular (LV) systolic dysfunction and followed for a median period of 4.5 years. We compared the impact of ramipril and matched placebo on occurrence of AF. The results were compared to similar trials.
Results: Over the 4.5 years follow-up, the incidence of new AF was low (2.1%, 177/8335), and ramipril did not significantly reduce the rate of new AF compared with placebo (86/4291 [2.0%] vs 91/4044 [2.2%]) with an odds ratio of 0.92 (95% confidence interval, 0.68-1.24; P = .57). These results added to the previous ACE inhibitor trials (excluding trials in patients with LV dysfunction) showed no significant reduction in new AF among patients treated with these agents (1088/20,930 [5.0%] vs 1343/22,878 [5.9%]; relative risk, 0.92; 95% confidence interval, 0.80-1.05).
Conclusion: Although the incidence of AF was low, treatment with ramipril in this population without known LV systolic dysfunction did not significantly reduce this dysrrhythmia.
Wednesday, October 24, 2007
buikomvang
Wellicht wist u het al: bij een buikomvang groter dan of gelijk aan 88 cm (voor vrouwen) en groter of gelijk aan 102 cm (voor mannen) is sprake van zogenaamde abdominale obesitas. Dit kenmerkt zich door vetophoping in de buik, te herkennen aan de appelvorm van de buik. Abdominale obesitas brengt meer gezondheidsrisico's met zich mee dan wanneer het vet op de heup en dijen zit, waarbij het lichaam meer de vorm van een peer heeft. Dit komt omdat het vet dat zich in de buikholte ophoopt, de vet- en suikerhuishouding van het lichaam in de war gooit. Hierdoor neemt het risico op hart- en vaatziekten toe. Bij de helft van de bezoekers van onze bus op de 50PlusBeurs constateerden wij een buikomvang boven de genoemde grenswaarden van 88 cm (vrouwen) respectievelijk 102 cm (mannen).
Saturday, October 20, 2007
statin
October 15, 2007 — Long-term follow-up of the West of Scotland Coronary Prevention Study (WOSCOPS) has now been published, and the results show that men prescribed statin therapy for five years during the clinical-trial period had fewer cardiovascular events a decade later, despite a large majority of the study cohort having stopped taking their cholesterol-lowering medication.[1]
Over the posttrial period, when treatment was in the control of the patients and their physicians, there remained a statistically significant reduction in death from coronary heart disease or nonfatal myocardial infarction (MI) among those treated with statin therapy compared with those treated with placebo, report investigators.
"In the original report of our study, we described a significant reduction in the risk of coronary events with the use of pravastatin," write lead author Dr Ian Ford (University of Glasgow, Scotland) and the WOSCOPS investigators in the October 11, 2007 issue of the New England Journal of Medicine. "We now report that during an extended follow-up period of approximately 10 years after the end of the trial, there was evidence of an ongoing reduction in the risk of major coronary events among study participants treated with pravastatin during the trial."
The results, say investigators, are presumably due to the stabilization of existing plaque and a slowing of the progression of coronary artery disease.
In an editorial accompanying the published study,[2] Dr Michael Domanski (National Heart, Lung, and Blood Institute, Bethesda, MD) writes that there should no longer be any doubt that the reduction of low-density lipoprotein (LDL)-cholesterol levels has a role in the prevention and treatment of disease.
"The central remaining que
Friday, October 19, 2007
calcium
Causes and Risk Factors of HypercalcemiaHypercalcemia is a relatively frequent medical problem. Its causes include increased intake (excess vitamin D or vitamin A, excess calcium intake), endocrine disorders (primary hyperparathyroidism, adrenal gland insufficiency), cancer (including multiple myeloma), and other causes (thiazide-diuretics, sarcoidosis, Paget's disease of bone).
Symptoms of HypercalcemiaMild hypercalcemia is often asymptomatic. Excessive urination and constipation are common. In more severe cases, kidney failure and coma can be observed. Cardiac arrhythmias (irregular heartbeats) may also occur.
Diagnosis of HypercalcemiaA blood test is necessary. An electrocardiogram (EKG) may also be done.
Treatment of HypercalcemiaSuccessful management of hypercalcemia requires management of the underlying disease. Variations in the cause of hypercalcemia and the extent to which hypercalcemia is a clinical problem factor into the decision-making process. Intravenous saline with intravenous furosemide (Lasix) is often adequate. In hypercalcemia of malignancy (cancer), more specific therapy is required including a bisphosphonate, which lowers blood calcium levels. If the hypercalcemic state is likely to be sensitive to steroids, the concurrent administration of calcitonin and glucocorticoids may be considered.
Questions To Ask Your Doctor About HypercalcemiaWhat is the cause of the hypercalcemia?
Is it related to hyperparathyroidism?
Is it the result of any other medical condition?
Where are the calcium deposits?
What drugs can be used to treat the hypercalcemia?
|
| 

|
calcium
zie: hypercalcemia wikipedia
calcium
Background: Hypercalcemia is a disorder that most commonly results from malignancy or primary hyperparathyroidism. Other causes of elevated calcium are less common and usually are not considered until malignancy and parathyroid disease are ruled out.
Hypercalcemic crisis does not have an exact definition, although marked elevation of serum calcium, usually more than 14 mg/dL, is associated with acute signs and symptoms of hypercalcemia. Treatment of the elevated calcium level may resolve the crisis.
The reference range of serum calcium levels is 8.7-10.4 mg/dL, with somewhat higher levels present in children. Approximately 40% of the calcium is bound to protein, primarily albumen, while 50% is ionized and is in physiologic active form. The remaining 10% is complexed to anions.
Pathophysiology: Plasma calcium is maintained within the reference range by a complex interplay of 3 major hormones, parathyroid hormone (PTH), 1,25-dihydroxyvitamin D (ie, calcitriol), and calcitonin. These 3 hormones act primarily at bone, kidney, and small intestine sites to maintain appropriate calcium levels.
Calcium enters the body through the small intestine and eventually is excreted via the kidney. Bone can act as a storage depot. The entire system is controlled through a feedback loop; individual hormones respond as needed to increase or decrease the serum calcium concentration.
For hypercalcemia to develop, the normal calcium regulation system must be overwhelmed by an excess of PTH, calcitriol, some other serum factor that can mimic these hormones, or a huge calcium load.
Hypercalcemia can result from a multitude of disorders. The causes are divided into PTH-mediated hypercalcemia and non–PTH-mediated hypercalcemia.
PTH-mediated hypercalcemia
Primary hyperparathyroidism originally was the disease of "stones, bones, and abdominal groans." In most primary hyperparathyroidism cases, the calcium elevation is caused by increased intestinal calcium absorption. This is mediated by the PTH-induced calcitriol synthesis that enhances calcium absorption. The increase in serum calcium results in an increase in calcium filtration at the kidney. Because of PTH-mediated absorption of calcium at the distal tubule, less calcium is excreted than might be expected. In PTH-mediated hypercalcemia, bones do not play an active role because most of the PTH-mediated osteoclast activity that breaks down bone is offset by hypercalcemic-induced bone deposition. Hypercalcemia of this disorder may remain mild for long periods because some parathyroid adenomas respond to the feedback generated by the elevated calcium levels.
Non–PTH-mediated hypercalcemia
Hypercalcemia associated with malignancy commonly is the result of multiple myeloma or breast or lung cancer and is caused by increased osteoclastic activity within the bone. Granulomatous disorders with high levels of calcitriol may be found in patients with sarcoidosis, berylliosis, tuberculosis, leprosy, coccidioidomycosis, and histoplasmosis. Iatrogenic disorders of calcium levels may increase secondary to the ingestion of many medications.
Frequency:
Mortality/Morbidity:
Sex:
Age:
Thursday, October 18, 2007
HDL cholesterol
|
|  Investigational Drug Dramatically Lifts HDL Cholesterol
By Ransdell Pierson and Bill Berkrot NEW YORK (Reuters) Oct 05 - An experimental Merck & Co. cholesterol drug from the same class as one that failed for Pfizer Inc had dramatic results in a small clinical trial, without the safety problems that doomed the Pfizer product. The drugs are inhibitors of the cholesteryl ester transfer protein (CETP), which regulates plasma HDL cholesterol concentration. At the second-highest dose tested in the 8-week trial, the Merck drug MK-859 raised levels of HDL by 139% and cut levels of LDL cholesterol by 40%, according to data presented on Thursday at the Drugs Affecting Lipid Metabolism meeting in New York. The Merck drug did not cause a rise in blood pressure, a side effect that had dogged Pfizer's now-abandoned torcetrapib and which many believe caused increased deaths associated with it. "As hard as we looked, we couldn't find any increase in blood pressure," said Daniel Bloomfield, a senior Merck research official who helped lead the study of MK-859. "The data really point out you can inhibit CETP with MK-859, and substantially reduce LDL and increase HDL, and importantly not raise blood pressure," he said. Four doses of the drug, 10 mg, 40 mg, 150 mg and 300 mg, were tested against placebo. "The data suggest any dose would be safe to go forward with" in any future late-stage trials, Bloomfield said. The incidence of side effects among patients taking MK-859, whatever the dose, was similar to those seen among patients given placebos. However, Bloomfield cautioned that his drug's true safety would not be known until it goes through much larger studies, which typically take 4 to 7 years and focus on the risk of heart attacks and death. Despite its impressive effects on LDL and HDL cholesterol, Bloomfield said Merck believes U.S. regulators are unlikely to approve MK-859 until such studies are completed. Merck's trial of MK-859 involved 589 patients with high levels of LDL cholesterol and/or low levels of HDL. HDL levels rose by 44% and 86%, respectively, among patients taking 10 mg and 40 mg doses of MK-859, while results seen with the highest 300 mg dose were similar to those of the 150 mg group. Levels of LDL cholesterol fell 16% and 27%, respectively, among those receiving the two smallest doses of the drug, and fell 39% in the highest-dose group. HDL levels rose only 4% in the placebo group, while LDL levels rose 2%. The Merck drug was also tested at all doses in combination with 20 mg doses of Lipitor. The combination did not have any additional impact on raising HDL, but it magnified the impact on LDL, lowering it as much as 68% at the highest dose of MRK-859.

Reuters Health Information 2007. © 2007 Reuters Ltd. Republication or redistribution of Reuters content, including by framing or similar means, is expressly prohibited without the prior written consent of Reuters. Reuters shall not be liable for any errors or delays in the content, or for any actions taken in reliance thereon. Reuters and the Reuters sphere logo are registered trademarks and trademarks of the Reuters group of companies around the world. | |

Tuesday, October 16, 2007
risk thrill gene
Young-Adult Gambling Is a Bad Bet!
College students who gamble also engage in a host of other risk behaviors.
Gambling among adolescents and young adults has become a significant public health problem. To examine gambling behavior in college athletes, researchers analyzed data from a 2003 National Collegiate Athletic Association risk-behavior study. The self-administered, anonymous survey was completed by 20,739 athletes (about 90% were aged 18–22).
Significantly more men than women reported gambling during the past year (62% vs. 43%). Based on a DSM-IV gambling screen, male athletes were significantly more likely than female athletes to be problem or pathologic gamblers (4.3% vs. 0.4%). In general, as gambling severity increased, so did the prevalence of substance use, disordered eating (particularly among females), and unprotected sex. Problem and pathologic gamblers also experienced significantly more drug and alcohol-related problems than nongamblers and social gamblers (participation in at least 1 of 14 gambling activities during the past year).
Comment: This study is the first national survey of gambling and risk behaviors among U.S. college athletes. Adolescents and college students are known to be at increased risk for gambling-related problems: Lifetime prevalence estimates of problem and pathologic gambling are more than twice that in adults. College students who gamble also engage in a host of other risk behaviors, including alcohol abuse, illicit drug use, and unsafe sex. The high prevalence of gambling among athletes and the link between gambling and other risk behaviors raises the question of whether school sports venues serve as pro-gambling environments — if so, that’s a far cry from conventional wisdom suggesting that athletic involvement is "just clean fun."
— M. Susan Jay, MD
Published in Journal Watch Pediatrics and Adolescent Medicine August 22, 2007
Citation(s):
Huang J-H et al. Gambling and health risk behaviors among U.S. college student-athletes: Findings from a national study. J Adolesc Heath 2007 May; 40:390-7.
Saturday, October 13, 2007
ablation in Thailand
Voeg een recensie toe
8.3
| Algemene indruk: | 9.0 |
| Prijs kwaliteits verhouding: | 9.0 |
| Klant vriendelijkheid: | 8.0 |
| Behandeling Ziekenhuis: | 8.0 |
| Professionaliteit Ziekenhuis: | 8.0 |
| Accomodatie: | 8.0 |
| Bedienend personeel: | 8.0 |
Algemene indruk: 9, Prijs kwaliteits verhouding: 8, Klant vriendelijkheid: 8
Friday, October 12, 2007
statins
 |  | | |  | | In This Article | | Introduction
 | | |  |  Overview: Recommendations of the Statin Safety Task Force and Benefit: Risk Considerations With Statin Therapy CME Posted 11/30/2006  Terry A. Jacobson, MD; James M. McKenney, PharmD |  Introduction Coronary heart disease (CHD) is responsible for more than 650,000 deaths among US residents annually and is the nation's number 1 cause of death.[1] It is also a leading cause of morbidity, resulting in approximately 700,000 new cases of myocardial infarction (MI) and 500,000 cases of recurrent MI annually.[1] The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or statins, are integral among CHD preventive therapies, significantly reducing the relative risk of coronary events and both coronary and all-cause mortality (vs placebo) by approximately 23% to 37% in several landmark clinical trials by virtue of their ability to modify lipid levels as well as inflammatory, thrombotic, and other mechanisms of atherosclerosis (Figures 1 and 2).[2-6]  | Figure 1. (click image to zoom) Primary and secondary prevention studies demonstrating risk reduction in coronary heart disease (CHD) with statin therapy. WOSCOPS = West of Scotland Coronary Prevention Study; AFCAPS/TexCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study; 4S = Scandinavian Simvastatin Survival Study; CARE = Cholesterol and Recurrent Events; LIPID = Long-Term Intervention with Pravastatin in Ischaemic Disease Reprinted with permission from National Lipid Association ( http://www.lipid.org/). |
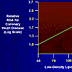 | Figure 2. (click image to zoom) Benefits of aggressive lipid-lowering therapy demonstrated by relationship between low-density lipoprotein cholesterol and relative risk for coronary heart disease. Reprinted with permission from National Lipid Association ( http://www.lipid.org/). |
Safety concerns about statins peaked after the voluntary worldwide withdrawal of cerivastatin (Baycol) in August 2001 because of a markedly increased reporting rate of fatal rhabdomyolysis (nearly 80 times higher than the rate for other statins available at that time).[7-9] Concerns increased after a petition was filed with the US Food and Drug Administration (FDA) concerning potential statin-related adverse effects.[10] Partly to address these concerns, the National Lipid Association (NLA) appointed a Statin Safety Task Force to evaluate statin safety, encompassing an assessment of the clinical literature; premarketing pharmaceutical data; spontaneous adverse event reports; meta-analyses of randomized clinical trials and analysis of cohort data; and an analysis of a large healthcare claims database.[11] In this article, we summarize the conclusions of the NLA Task Force and provide recommendations to health professionals who manage primary and secondary coronary prevention using statins. To contextualize the safety data, we also discuss the benefits of statin treatment vs the risk of adverse effects in terms of the number of clinical events per person-year of statin treatment. | | 
|
| Section 1 of 7 | |  Terry A. Jacobson, MD, Professor of Medicine, Emory University; Director, Office of Health Promotion and Disease Prevention, Grady Health System, Atlanta, Georgia
James M. McKenney, PharmD, Professor Emeritus, Virginia Commonwealth University, President and Chief Executive Officer, National Clinical Research, Inc., Richmond, Virginia
David Good, Editorial Director, Medscape Cardiology
Ariana Del Negro, Associate Editorial Director, Medscape Cardiology
Disclosure: Disclosure: Terry A. Jacobson, MD, has disclosed that he has served as an advisor or consultant to AstraZeneca, Merck, Pfizer, Schering-Plough, and Novartis.
Disclosure: Disclosure: James M. McKenney, PharmD, has disclosed that he has received grants for clinical research from AstraZeneca, GlaxoSmithKline, KOS, Merck, Pfizer, Roche, Schering-Plough, and Takeda. He has also disclosed that he is on the Speaker's Bureau for AstraZeneca, KOS, Merck, Pfizer, Reliant, and Schering-Plough. Dr. McKenney has also disclosed that he is a consultant to AstraZeneca, KOS, Microbia, Pfizer, and Sankyo.
Disclosure: David Good has disclosed no relevant financial relationships.
Disclosure: Ariana Del Negro has disclosed no relevant financial relationships.
 Medscape Cardiology. 2006; 10(2):. ©2006 Medscape  | |
|
|  | 
|







